
enrique.chacon@thinkvaccines.org


“COVID-19 proved that either individual or even country-based decisions during a pandemic are insufficient, causing vaccination inequities, and substantially led to a higher spread of new variants. Immunization efforts should be done from a global perspective, with regional adaptations.”
ENRIQUE CHACON-CRUZ
CEO / Founder
mission
To counsel, and participate on education, information, and scientific spreading of vaccines and vaccination, as well as advice in clinical trials, and epidemiological surveillance, leading to a world with lesser morbidity/mortality associated to vaccine-preventable-diseases.
Why Choose think vaccines ?
ADVISORY IN CLINICAL DEVELOPMENT
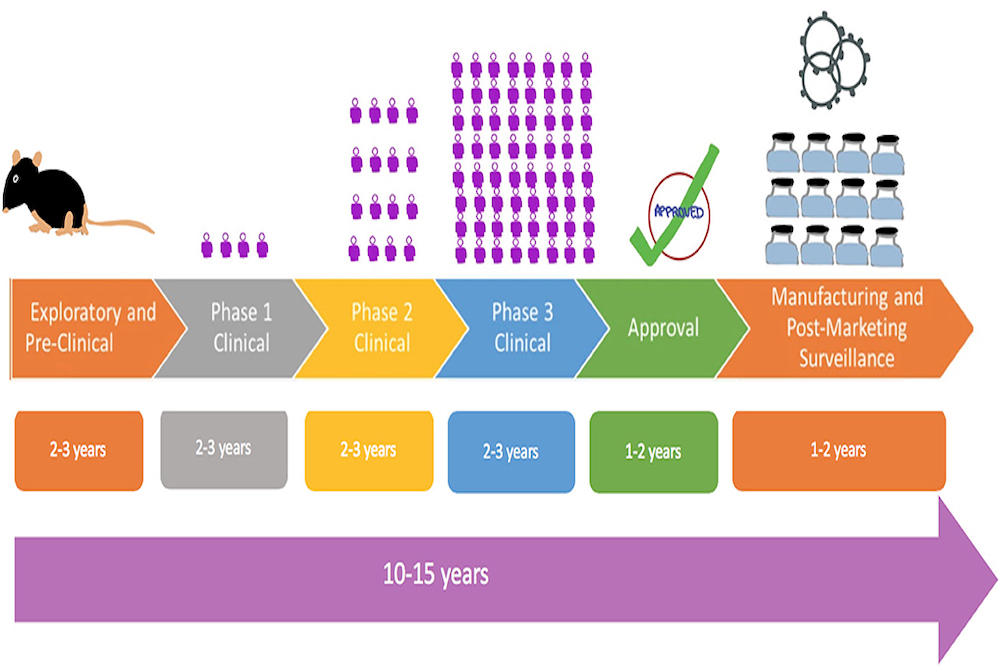
Clinical development for vaccines
(VCD) differs significantly from molecules and drugs,
and currently, since COVID-19, VCD is playing a critical role.
Think Vaccines aims to aid to better control (and even eradicate or eliminate) current diseases where vaccines are available,
begin and release new vaccines,
to strategize platforms for development and prioritize strategies against current and new emerging pathogens with potential for an epidemic or pandemic.
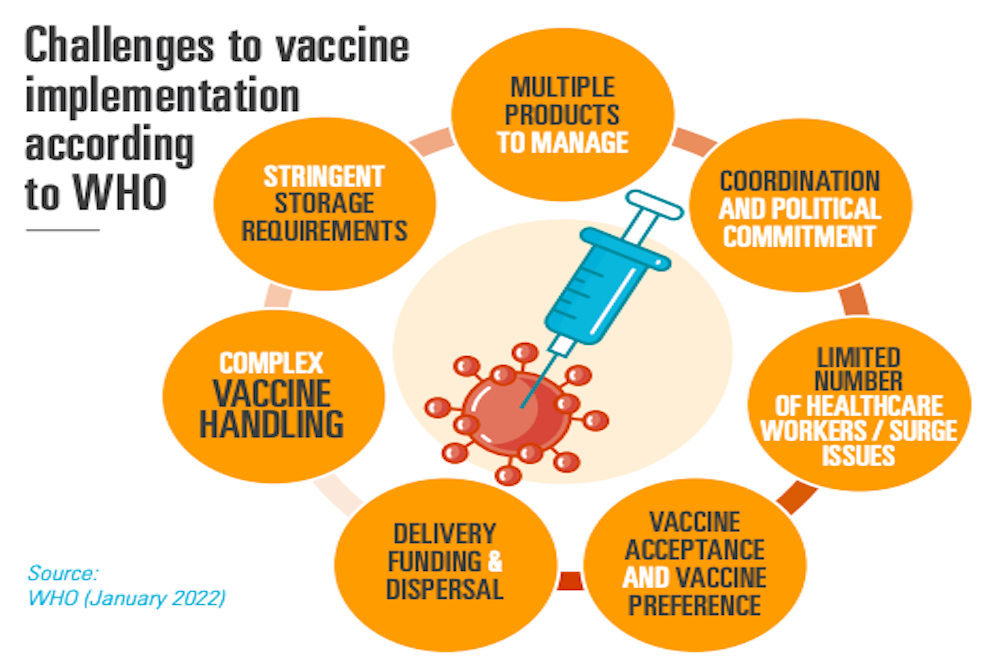
COUNSELING FOR VACCINE IMPLEMENTATION
The global epidemiology of infectious diseases (ID) varies from region to region, as the disease burden, different subtypes of pathogens, seasonality, and age-related attack rates. Moreover, for various known ID, more than one option for preventive vaccines is available. Vaccine implementation, therefore, should be advised by regions and supported by a health-economic evaluation; in which we can provide advice and counseling taking into consideration all the influencing factors.

AIDING TO IMPROVE EPIDEMIOLOGICAL SURVEILLANCE
The lack of surveillance for infectious diseases (ID) has led many times to the erroneous assumption that “X disease” is not a public health problem. The latter has historically led to unexpected outbreaks with its consequential burden on both morbidity/mortality, and cost. Implementation of effective epidemiological surveillance for ID is a mandatory tool to better define both a disease and economic burdens. Active surveillance methods are encouraged; however, their methodology could be costly and complex, hence, we can provide regional-based counselling.

EXPANDING THE KNOWLEDGE OF VACCINOLOGY: EDUCATION, AND MORE
VACCINOLOGY IS NOT JUST VACCINES. It is a journey, starting from the discovery of a prototype, in vitro studies, animal experimentation, human clinical trials, approval, launching, and post-approval studies. This long and extremely complex process involves basic science, investment, manufacturing, pharmacovigilance, a clinical development plan (per clinical trial), pharmacovigilance, community implementation, etc., all of which require from all sorts of health care providers, legal institutions, stakeholders, governments, facilities, engineers, among many others.
We offer a multidisciplinary course in basic vaccinology, directed to everyone involved in this science (medical doctors, pharmacists, veterinarians, nurses, policy makers, PhDs, public health specialists, lawyers, epidemiologists, laboratory workers, etc.). In addition, as part of this necessary knowledge, and accounting a vast experience in the field, we have participated, and will keep contributing to all vaccines-related studies for publication.


Science is based on evidence, trust vaccines, respect the rules but do not hesitate to ask. The best vaccines are the ones you can get, and build a better world.






